
Sleep Restore Based On EMDR
Overcome the Stress that’s keeping you awake
Sleep Restore App Study
A randomized wait-list controlled study of the use of an app based on EMDR to manage stress-related insomnia
Mark Grant MA , Richard Lau, Shannen Aungiers
Study Objectives:
Insomnia is a common health problem. Emergency workers are much more prone to sleeping problems than people in general, because of the unique demands of their work (e.g., shift-work, restricted sleep during emergencies etc). Insomnia in emergency workers is also often associated with other health problems such as PTSD, depression and impaired cognitive performance. Sleep apps are an increasingly popular solution to insomnia although most are not specifically designed to address stress-related insomnia and very few have been tested under research conditions. Sleep Restore was developed to specifically address stress-related insomnia through a combination of tailor-made guided meditations and bilateral stimulation, a treatment element of EMDR which can alleviate insomnia associated with PTSD. The aim of this study was to test the efficacy of this app for the management of insomnia in emergency workers suffering from PTSD or PTSD symptoms.[1]
Participants
Inclusion Criteria
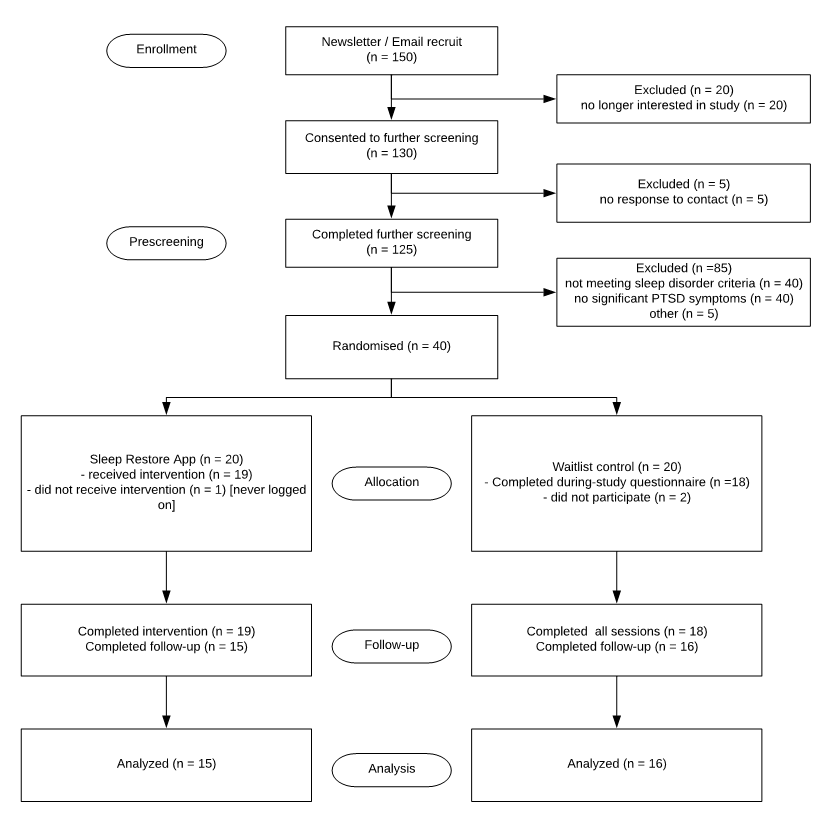
Study participants will be recruited from 1/10/19 to 1/10/20 through internal postings and promotion within the fire brigade and police department in Australia. Study participants will include individuals who (1) complain of sleeping problems of any kind (trouble falling asleep, night-waking, lack of restorative sleep) as evaluated by meeting the Sleep condition indicator (SCI) criteria for insomnia and/or (2) PTSD symptoms/diagnosis at the time of self-report screening based on responses to the PCL-C checklist.
Exclusion criteria
Exclusion criteria will be (1) history of major psychiatric disorder associated with insomnia (eg; bipolar disorder) and/or PTSD; (2) current exposure to concurrent traumatic event(s); (3) major on-going health problems (e.g. spinal injury requiring surgery) (4) unstable housing. Diagnosis of sleep apnea will be recorded into medical condition history but the subject will not be excluded from the trial. Participants involved in worker-compensation claims will not be excluded, but cases will be noted as a variable given the stressful nature of the circumstance.
Study design
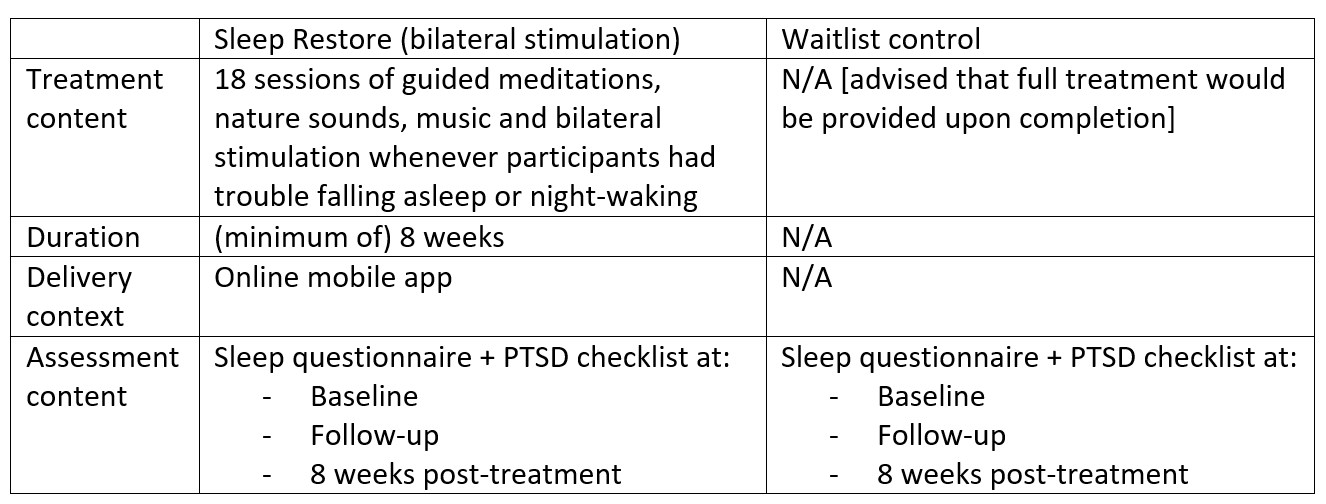
The design of the present study is a parallel-group randomized controlled trial comprised of (1) an 8-week Sleep restore app treatment arm and (2) an 8-week waitlist control arm. With blind assignment by a computer-generated random allocation schedule, half of the participants will be randomized to the treatment group and the other half to the waitlist control group. Self-report measures of sleep quality, sleep behaviors, PTSD symptoms and medication use will be obtained in all participants at baseline, during-trial, and 8 weeks post-treatment. Participants allocated to the waitlist control group will be provided the full version of sleep restore app and treatment materials upon completion of the study. All data collection, treatment and assessment procedures will be conducted online and managed electronically. All experimental procedures were approved by the Human Research Ethics Department of the NHMRC, and informed consent was obtained from all participants.
Assessment measures
All assessment materials will be provided in the form of online surveys that requires an internet browser on any device. These materials aim to assess which stress factors are affecting sleep and whether or not the user might be suffering from a medical sleep disorder such as sleep apnea or restless leg syndrome. Subjects will be advised to seek professional help through email if they report positive in the above condition.
PTSD Checklist
The PCL-C is a validated self-report scale for the assessment of PTSD symptoms with a total of 17 items on a 5-point scale correspond to DSM-IV symptoms of PTSD. Checklist scores range from 17 to 85, with a score of 30-35 being the cut-off for a diagnosis of PTSD and higher score indicating a higher severity of PTSD symptoms.
Sleep Condition Indicator
The Sleep Condition Indicator (SCI) is a validated 8-item self-report measure of sleep quality based on Insomnia Disorder criteria of DSM-V. The list generates scores in the range of 0 to 32, which can be converted to a 0 to 10 format, with a higher score indicating better sleep condition.[2]
Treatment conditions
Sleep Restore App
Sleep Restore was developed to specifically address the effects of stress which lead to insomnia, namely: trouble falling asleep, night-waking, hypervigilance, worry, nightmares and feelings of lack of safety. Based on the response collected from each participant in the online assessment, the Sleep Restore app will automatically generate a unique meditation playlist from 18 sessions of pre-installed guided materials including natural sounds, music and bilateral stimulation, individually designed to address the different effects of stress. Materials will be available immediately after completion of baseline assessments. Participants will be instructed to utilise these materials whenever they experience trouble falling asleep. They will also be encouraged to revisit the app more often in order to unlock extra treatment materials.
Materials with verbal content are intended to retrain the user’s brain towards a relaxed state to help inducing sleep, while non-verbal materials that do not involve cognitive stimulation are designed for overcoming night-walking. Furthermore, the incorporation of bilateral stimulation as part of the materials is expected to decrease sleep related stress and arousal, in turn encouraging a restful sleep.
Waitlist control
The waitlist control condition will consist of only monitoring of sleep quality and PTSD symptoms using the assessment measures during assessment periods at baseline, follow-up and 8 weeks post-study. Participants will not be provided any treatment materials or feedback until the end of study.
[1] Talbot, L. S., Maguen, S., Metzler, T. J., Schmitz, M., McCaslin, S. E., Richards, A., … & Varbel, J. (2014). Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep, 37(2), 327-341.
[2] Espie, C. A., Kyle, S. D., Williams, C., Ong, J. C., Douglas, N. J., Hames, P., & Brown, J. S. (2012). A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep, 35(6), 769-781.
Treatment condition summary
Protocol standardisation
Participants of both treatment conditions will be invited to commit to (or dropout of) the trial following the consent procedures. All treatment and assessment procedures will be scripted and automated online to assure integrity and fidelity of treatment, as well as the mode of delivery of the intervention. All interactions will be electronically recorded with time-stamped data for particular activities such as assessment responses and the use of treatment materials.
Statistical Analysis
Data analysis will be carried out under 80% power to detect a medium effect size at P = .05 using SPSS statistical software. These criteria implied a proposed sample size of 150, randomised half to the treatment group and the other half to the waitlist control group. All tests were planned and run using 2-sided tests of significance, with P < 0.05 considered statistical significance. Repeated-measures ANOVA was carried out on assessment measures collected at baseline, immediate post-treatment and 8 weeks post-treatment for both conditions. Analysis of covariance (to control for baseline score) was conducted to test differences at baseline and post-treatment. Paired t-tests were conducted for the treatment group to compare baseline results to 8 weeks post-treatment data.
